Listeria monocytogenes is a gram-positive, facultative anaerobic foodborne pathogen. Eating food contaminated with Listeria can cause Listeriosis, a life-threatening infection that mostly affects older adults, immunocompromised people, and pregnant women and their newborns.¹ The psychrotrophic pathogen has resulted in some major foodborne disease outbreaks in the United States in recent years.² As Listeria monocytogenes is ubiquitously present in nature, its presence in fresh produce, as well as raw food ingredients, cannot be avoided. These ingredients when used for food manufacturing purposes may lead to Listeria cross-contamination in the manufacturing facilities and eventually end up contaminating the food items. Hence, environmental Listeria control in the food manufacturing plants has been a topic of great importance for food safety managers. A typical food manufacturing process consists of multiple steps and uses different temperature and processing conditions such as cold and moist operational settings. Some of these conditions may turn out to be conducive for Listeria growth.
Also, the pathogen can exhibit various stress responses and forms biofilm under unfavorable environmental conditions,³ which makes it much more difficult to control as these conditions make bacteria more resistant and tolerant to physical and chemical treatments. Hence, to control listeria cross-contamination issues, it is important to not only understand the ever-evolving characteristics of this pathogen but to also focus on the influence of various treatments and conditions on its survival during processing and post-processing stages.
Over the years, several foods have been linked to outbreaks and multiple cases of Listeriosis.4 Since most of these products had extended shelf-lives, the sporadic outbreaks have drawn attention to the importance of time-temperature monitoring during processing, transportation, and storage of ready to eat food products.5 It is well known that Listeria can enter into an injured state when exposed to several processing treatments such as sublethal heating and freezing, drying, or exposure to chemicals.6 Also, several reports in the past have indicated that Listeria monocytogenes could potentially survive minimal thermal processes if present in high numbers or if the background flora in the product is high enough to bypass the thermal treatment. Besse,7 in his review, stated that it was not possible to eliminate all bacteria with minimal heat treatment, as it could lead to a physiological stress state within cells, leaving them injured. Upon reversal to the favorable conditions, the injured cells could repair themselves and pose a threat to product safety and quality. Hence, a failed attempt to consider their recovery potential in a particular food can lead to underestimation of their true incidences.6
As a part of my doctoral dissertation, I evaluated the potential presence of heat-injured cells in ice cream matrix when exposed to minimum heat treatment, by employing direct plating as well as FDA- approved enrichment protocol. For the ice cream challenge studies, the direct plating on Modified Oxford Agar did not pick up any survivors, however, when these heat-treated samples were passed through BLEB enrichment step,8 random heat-injured cells were detected at the highest dose level of 4+log. Hence, through this study, the level of cross-contamination (dose) emerged as a predictor of the potential presence of heat-injured cells of Listeria.9 The study also highlighted the significance of using enrichment protocol to enumerate any random presence of heat-injured cells. In continuation, I evaluated the possible protective role of the ice cream mix matrix to explain the random presence of potentially heat-injured cells. The scanning electron micrographs of the heat-treated ice cream mix samples showed cells entrapped within the larger air pockets of ice cream mixes (Fig. 1) suggesting that Listeria cells can be entrapped within the larger air pockets of ice cream mix and may receive an inadequate thermal effect, resulting in their detection as potentially heat-injured cells.10 I further accessed the recovery potential of heat-injured cells of Listeria innocua within the ice cream mix matrix itself under the mix aging and storage conditions. It was interesting to note that none of the pasteurized ice cream samples showed recovery of any heat-injured cells, under the experimental conditions.
Yet another important aspect related to injured cells of Listeria in a product is their potential recovery in the host’s GI tract. As upon consumption of food containing injured cells, there is a potential that these cells can withstand the host’s barriers. Hence, I further investigated the recovery potential of injured cells of Listeria innocua using simulated gastrointestinal fluids.11 The ice cream samples containing potentially injured cells of Listeria innocua were sequentially passed through simulated gastric and intestinal fluids. The direct plating in this case, again, did not detect any cells however, the injured cells were detected by the BLEB enrichment protocol after the exposure to gastric fluid. This confirmed the inability of injured cells to recover during exposure to gastrointestinal fluid. However, the potentially injured cells were still present in the gastric fluids.
The potentially heat injured cells did not recover in the ice cream mix as well upon exposure to gastrointestinal fluids under the conditions of the challenge studies their mere presence in ice cream mix and gastric fluid may pose a risk to immunocompromised and high-risk group people if recovered under any circumstances.
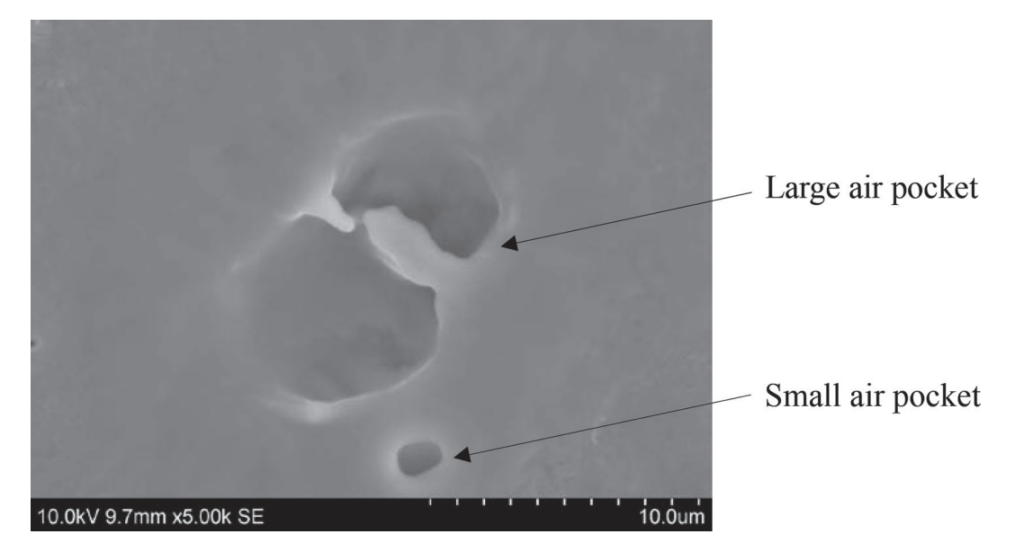
Fig. 1: Scanning electron microscopy image depicting heat injured cells of Listeria innocua entrapped in a large pocket of spiked ice cream mix (Source: https://www.journalofdairyscience.org/action/showPdf?pii=S0022-0302%2819%2930708-8)
References
¹ Roberts, B. N., Chakravarty, D., Gardner, J. C., 3rd, Ricke, S. C., & Donaldson, J. R. (2020). Listeria monocytogenes Response to Anaerobic Environments. Pathogens (Basel, Switzerland), 9(3), 210.
² Listeria Outbreaks. (2020, October 23). Retrieved February 01, 2021, from https://www.cdc.gov/listeria/outbreaks/index.html
³ Colagiorgi, A., Di Ciccio, P., Zanardi, E., Ghidini, S., & Ianieri, A. (2016). A Look inside the Listeria monocytogenes Biofilms Extracellular Matrix. Microorganisms, 4(3), 22.
4 Robert L. Buchanan, Leon G.M. Gorris, Melinda M. Hayman, Timothy C. Jackson, Richard C. Whiting, A review of Listeria monocytogenes: An update on outbreaks, virulence, dose- response, ecology, and risk assessments, Food Control, Volume 75, 2017.
5 Jeffrey M. Farber, Gosia K. Kozak, Suzanne Duquette, Changing regulation: Canada’s new thinking on Listeria, Food Control, Volume 22, Issue 9, 2011
6 Donnelly C. W. (2002). Detection and isolation of Listeria monocytogenes from food samples: implications of sublethal injury. Journal of AOAC International, 85(2), 495–500.
7 Besse, N. (2002). Influence of various environmental parameters and of detection procedures on the recovery of stressed L. monocytogenes: A review. Food Microbiol. 19:221–234.
8 FDA. 2017. Detection of Listeria monocytogenes in foods and environmental samples, and enumeration of listeria monocyto- genes in foods. Chapter 10 in Bacteriological Analytical Manual (BAM). Accessed Aug. 15, 2018. https://www.fda.gov/food/ foodscienceresearch/laboratorymethods/ucm071400.htm.
9 Neha N, Anand S, Djira G, Kraus B, Sutariya S. Listeria cross-contamination levels in raw ice cream mix can serve as a predictor of their potential presence as heat-injured cells. J Dairy Sci. 2018 Nov;101(11):9659-9669.
10 Neha, N., & Anand, S. (2019). Short communication: Entrapment of Listeria cells within air pockets of ice cream mix matrix may lead to potentially heat-injured cells. Journal of dairy science, 102(11), 9721–9726.
11 Singh, N., Anand, S., Kraus, B., & Sutariya, S. (2021). Short communication: Evaluating the recovery potential of injured cells of Listeria innocua under product temperature-abuse conditions and passage through simulated gastrointestinal fluids. Journal of dairy science, S0022-0302(21)00068-0.



